Abstract
Patients with myeloproliferative neoplasms (MPN) frequently carry a somatic mutation in the JAK2 gene (JAK2-V617F), which causes an overactivation of the JAK-STAT signaling pathway that leads to erythrocytosis, thrombocytosis and/or myelofibrosis. JAK2-V617F is acquired in a hematopoietic stem cell (HSC). Therefore, in order to develop novel effective therapies, it is imperative to specifically target the mutant HSCs, as these cells are the reservoir that maintains MPN. To date, pegylated-interferon-alpha (pegIFNα) is the only treatment known to induce molecular remission in some patients with MPN. The mechanism behind this effect is not completely understood, but recent studies suggest that pegIFNα selectively pushes mutant HSCs into cell cycle, which leads to their differentiation and exhaustion.
We hypothesized that disrupting components of the negative feedback loop to JAK-STAT signaling in HSCs would lead to JAK-STAT hyperactivation and thereby sensitize HSCs to the effects of pegIFNα. To investigate which genes are governing the feedback loop, we first performed single-cell RNA-sequencing on HSCs from our JAK2-V617F MPN mouse model. We found that Socs2, a JAK-STAT negative regulator gene, was one of the top upregulated genes in MPN HSCs, while expression of other genes known to be involved in JAK-STAT negative feedback loop was unaltered by the JAK2 mutation.
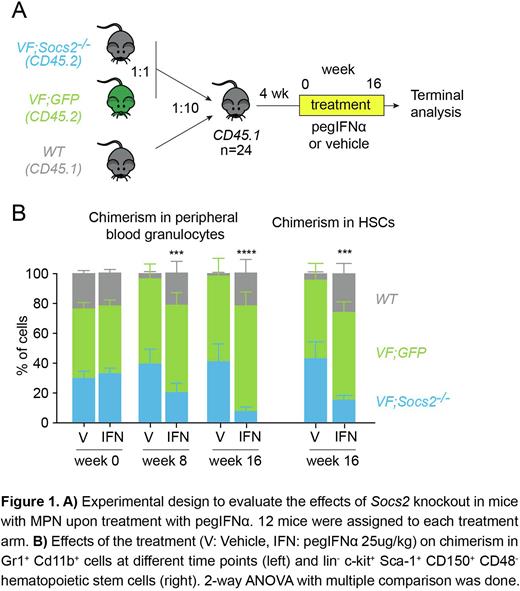
We examined the effects of loss of Socs2 by crossing our JAK2-V617F mice (VF) with a constitutional Socs2 knockout strain (Metcalf et al. Nature, 2000), to obtain VF;Socs2-/- double mutant mice. We performed competitive bone marrow transplantations by mixing bone marrow cells from VF mice that also express a GFP reporter gene (VF;GFP) at 1:1 ratio with bone marrow cells from VF;Socs2-/-mice, and added a 10-fold excess of bone marrow cells from wildtype CD45.1 mice. This 1:1:20 mixture was then transplanted into lethally irradiated wildtype CD45.1 recipient mice. These mice were allowed to engraft for 4 weeks and were then treated for 16 weeks with pegIFNα (25 ug/kg s.c. once weekly) or vehicle (Figure 1A). CD45.2 was used to track total VF plus VF;Socs2-/- chimerism and GFP distinguished between VF;GFP and VF;Socs2-/- cells.
The ratio between VF;GFP and VF;Socs2-/- chimerism remained stable in the vehicle group during the course of the experiment (Fig.1B). However, the VF;Socs2-/-chimerism was reduced in the pegIFNα-treated mice, both in peripheral blood granulocytes (7.6% compared to 41% in the vehicle group) and in the bone marrow. Specifically, HSC chimerism of double mutant cells dropped from 43% in the vehicle arm to 15% in the treated mice (Fig.1B). We also performed transplantation of unfractionated BM cells from VF;Socs2-/- or VF mice in competition with BM from GFP-expressing wildtype mice at a ratio of 1:10. These transplanted mice were randomized and treated for 16 weeks with either pegIFNα or vehicle (n=12 per arm). Although both single and double mutant groups had similar hematologic responses, the VF;Socs2-/-arm had a better molecular response than VF (data not shown). 41% of the mice from the double mutant group had molecular response in peripheral blood and in HSCs, compared to only 16% in the VF group.
Our data show that releasing the brake applied by Socs2 on Jak2 signaling magnifies the pegIFNα-induced effects on VF;Socs2-/- HSCs, leading to a better molecular response. Thus, hyperactivating JAK2-STAT signaling in HSCs by inhibiting Socs2 could potentially improve the molecular response caused by pegIFNα.
Disclosures
Skoda:Ajax Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene and Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal